Results With SUNLENCA®
See how SUNLENCA worked in a clinical study
SUNLENCA was studied in adults with HIV that was resistant to other HIV medications
A study was conducted to find out if adding SUNLENCA to other HIV medications helped people significantly decrease the amount of HIV virus in their blood in 2 weeks (15 days) and reach and stay undetectable (less than 50 copies/mL of the HIV virus in the blood) after 6 months (26 weeks) and 12 months (52 weeks) of treatment.
72 adults
with detectable levels of HIV-1 that was resistant to other HIV-1 medicines. The adults chosen for the study had a limited amount of remaining treatment options to try.
Participants chosen had:
- A viral load of ≥400 copies/mL
- Resistance to at least 2 medications from each of at least 3 different HIV treatment classes
- Two or fewer HIV-1 medications available to them without resistance, intolerability, drug access, or other safety concerns
START OF STUDY
GROUP 1 36 adults
Some adults in Group 1 added SUNLENCA right away. Others added a placebo for 2 weeks before swapping the placebo for SUNLENCA. After 2 weeks, participants in Group 1 were receiving SUNLENCA in addition to taking their other HIV-1 medicines as prescribed by their doctors.*
12 adults started with a PLACEBO
Some of the adults in Group 1 were given placebo tablets in addition to their other HIV-1 medicines.
24 adults added SUNLENCA
Some of the adults in Group 1 were given SUNLENCA tablets right away in addition to their other HIV-1 medicines.
2 WEEKS (15 DAYS)
After 2 weeks, participants were receiving SUNLENCA injections every 6 months in addition to their other medicines.*
6 MONTHS/1 YEAR (26 WEEKS/52 WEEKS)
Participants received SUNLENCA injections every 6 months in addition to their other HIV-1 medicines.†
*People who started with a placebo took SUNLENCA tablets in addition to their other HIV-1 medicines for 2 weeks before starting SUNLENCA injections.
†During the trial, many participants also took oral SUNLENCA temporarily (one 300-mg tablet once every 7 days) when they couldn't receive their every-6-month injection on time.
SUNLENCA helped to quickly decrease the viral load in most people after just 2 weeks
In Group 1, 88% of adults (21 of 24 people) had less HIV virus in their blood after adding SUNLENCA and continuing to take the other HIV medicines that were prescribed by their healthcare providers.
88%
of adults had less HIV virus in their blood 15 days after adding SUNLENCA, as compared to 17% (2 of 12) of adults on placebo.
What were the side effects with SUNLENCA?
The most common side effects seen with SUNLENCA, experienced in at least 3% of the people in the clinical study through Week 52 (1 year), were nausea (4%) and injection site reactions (65%). The majority (96%) of the side effects associated with SUNLENCA were mild or moderate in severity.
- Through 1 year of the clinical trial, 1 person stopped taking SUNLENCA due to an injection site reaction. No other participants stopped taking SUNLENCA due to side effects through 1 year.
These are not the only possible side effects of SUNLENCA. Tell your healthcare provider if you have any side effects that bother you or do not go away.
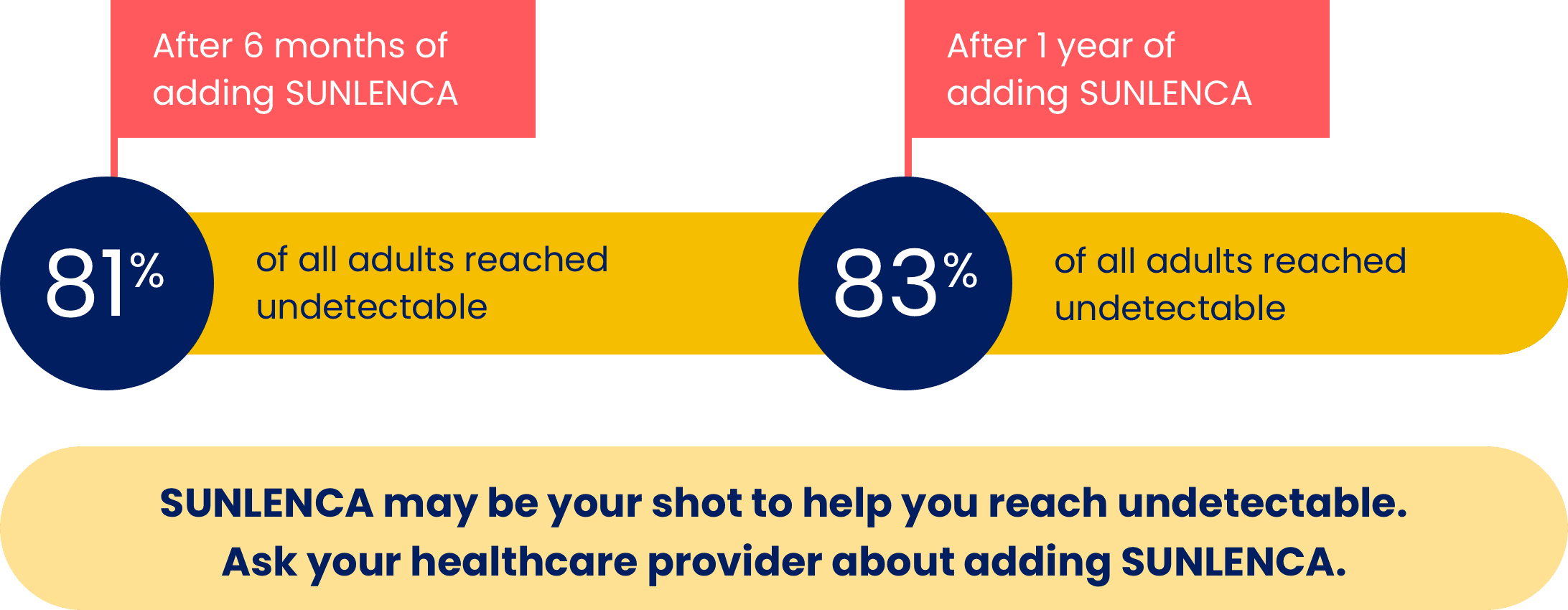
SUNLENCA helped adults reach undetectable, and most adults remained undetectable after 1 year
In Group 1, 81% of adults (29 of 36 people) who added SUNLENCA and continued to take the other HIV medicines that were prescribed by their healthcare provider reached undetectable (less than 50 copies/mL of the HIV virus in the blood) by the 6-month mark. After a year, 83% of adults (30 of 36 people) were still undetectable.